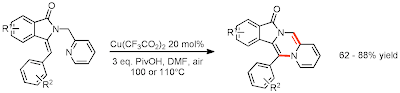
Screening of copper catalysts with acid additives resulted in the following set of conditions in 8-48 hours, which allow for fluoro, chloro, bromo, and ether groups appended to the aryl rings... I'd like to have seen a broader substrate scope at least addressed in the paper.
The inclusion of a potential mechanism was obviously included just out of necessity in a methods paper, and the arrows are pretty much what I could make of it. The pyridinium nitrogen draws copper in close, bites it, and isomerizes allowing the Cu-N bond to do some acrobatics and add across the double bond, followed by rearomatization and elimination of the barely-catalytic copper (I am of the school that views 20% as sub-stoichiometric, NOT catalytic). But! My purpose in including this paper in this post was to demonstrate the cool stuff that copper can do that we'd normally just assume palladium would be used for, and to comment that I'd be interested to see what, if any, biological activity these structures offer. Each day this week (or over the next 2 weeks) I will address a different transformation via copper catalysis.
- Lu J, Jin Y, Liu H, Jiang Y, & Fu H (2011). Copper-Catalyzed Aerobic Oxidative Intramolecular Alkene C-H Amination Leading to N-Heterocycles. Organic letters PMID: 21696194